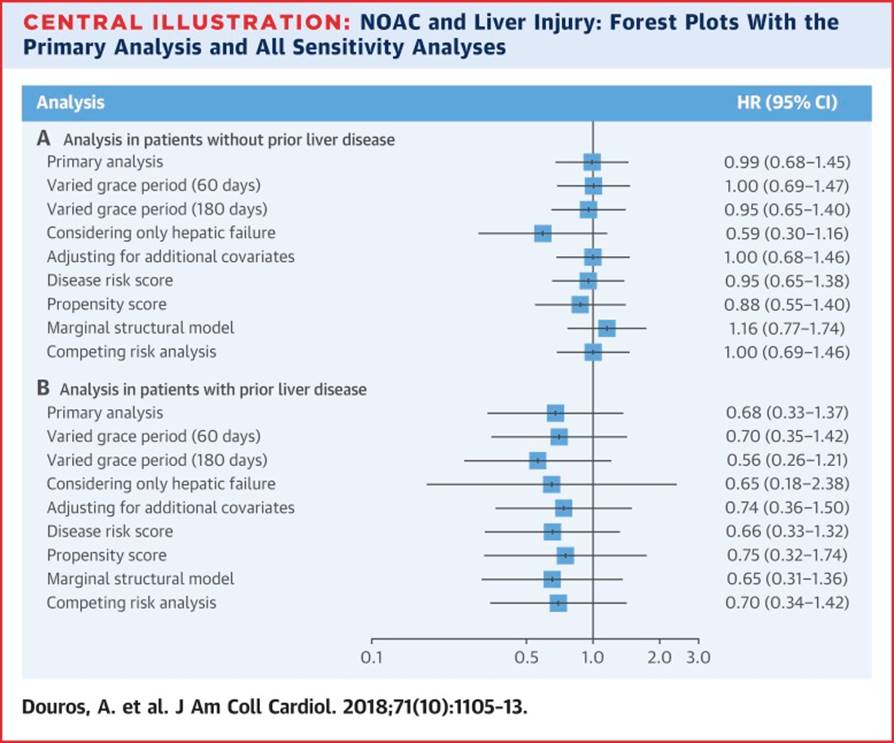
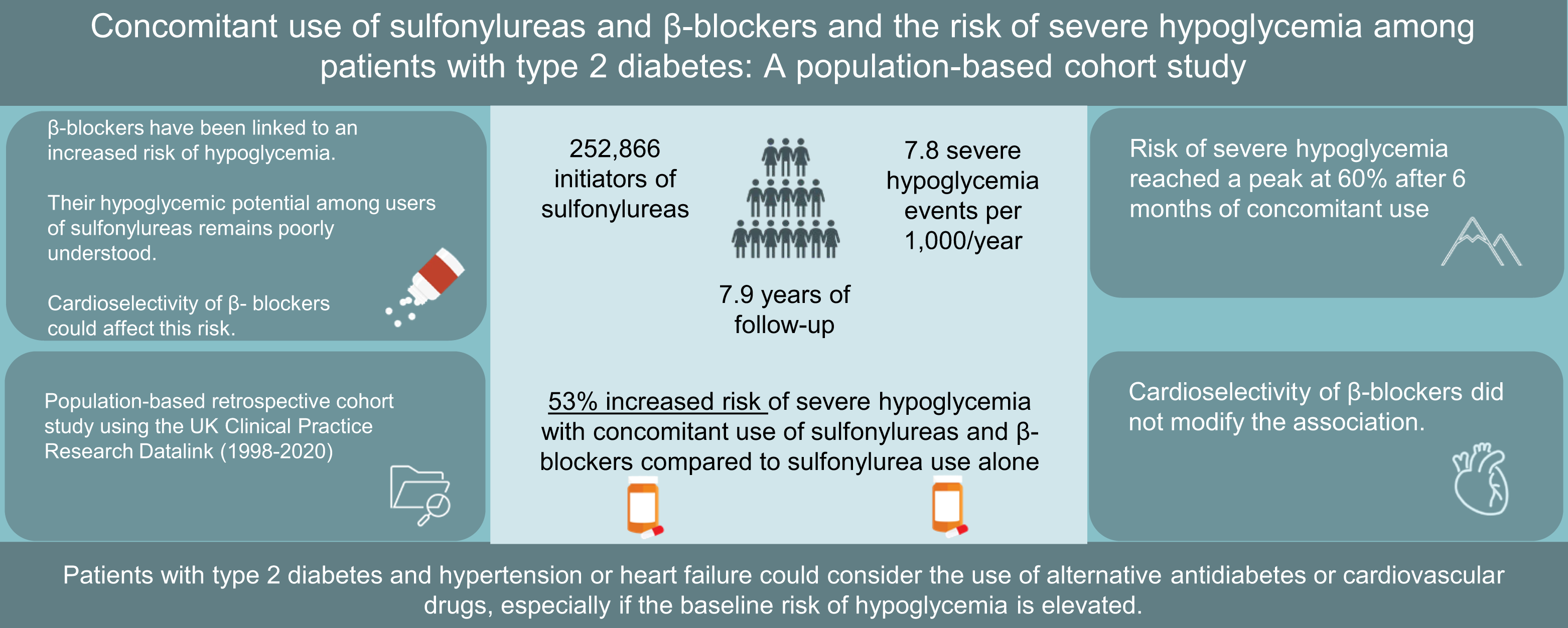
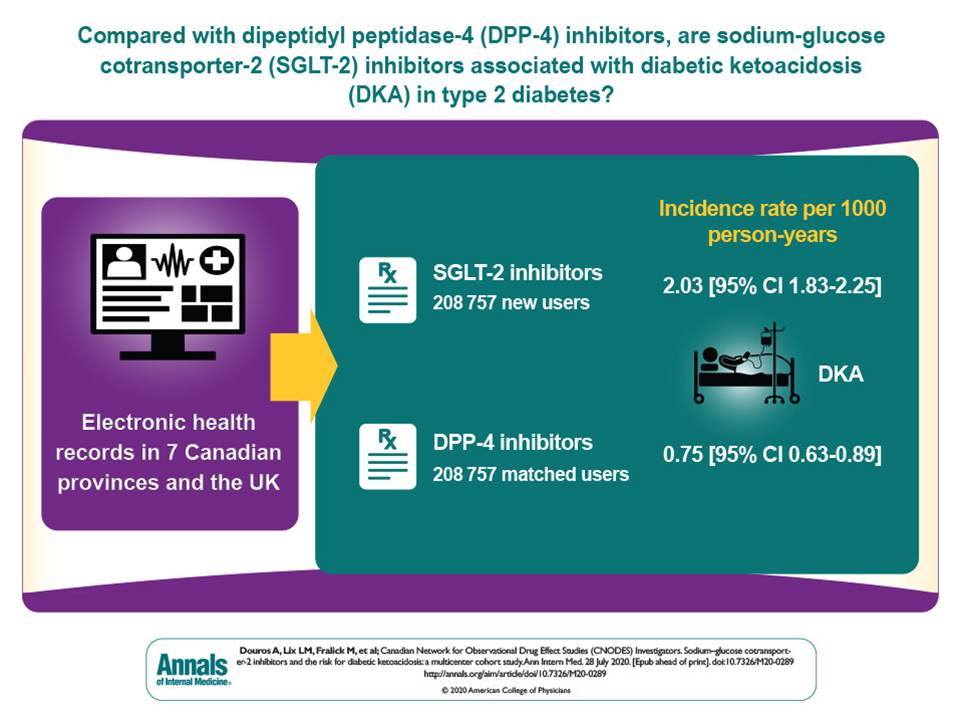
You can check out my complete list of publications on PubMed.
To date, my research has resulted in 78 peer-reviewed publications (36 as first author, 14 as senior author). Several of these publications have appeared in top-tier journals including the British Medical Journal (4x), Annals of Internal Medicine, European Heart Journal, Journal of the American College of Cardiology, Diabetes Care (7x), Alzheimer’s & Dementia, JACC: Heart Failure, and Neurology. Moreover, my publications have been the subject of 10 editorials.
Below are a few highlights of my published work.